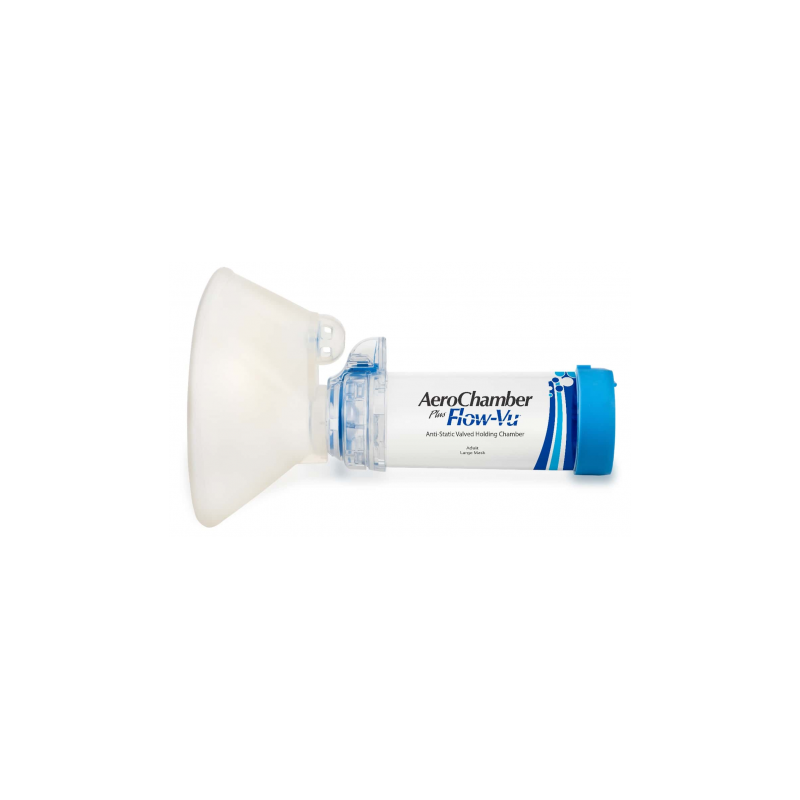
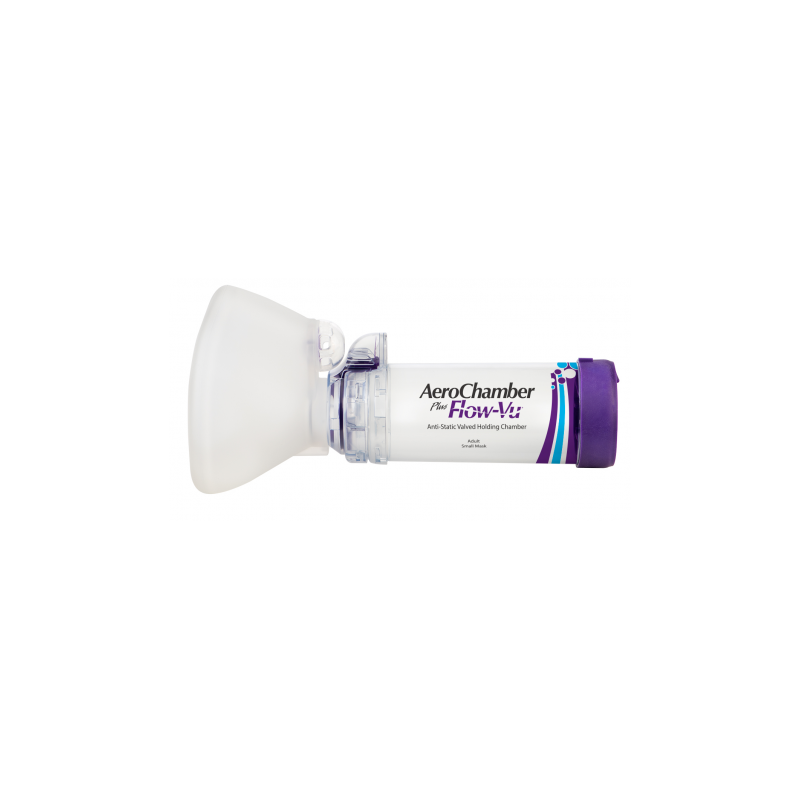
Anti-Static valved holding chamber with mouthpiece for adult
Anti-Static Valved Holding Chamber for use with a metered dose inhaler or soft mist inhaler.
Helps deliver inhaler medicine to the lungs1.
Medical device
Intended use: This chamber is intended to be used by patients who are prescribed a metered dose inhaler or soft mist inhaler, to deliver the aerosol medication from the inhaler to your lungs.
The clinical benefit of chambers is to improve the coordination of inhaling your medication. This is achieved by holding the medication, allowing you more time to inhale while filtering out the larger particles that would not reach your lungs.
If you have any concerns regarding the use of the chamber or its delivery of your inhaler medication, please consult a healthcare professional.
BEFORE EACH USE
This anti-static chamber can be used directly out-of-package. Ensure these instructions and the instructions supplied with the inhaler have been read and are kept available at all times. Carefully look into the chamber. If there is any visible dust or other debris inside, then clean the chamber. Replace immediately if it is damaged or has missing parts.
FOR USE WITH METERED DOSE INHALERS (PUFFERS)
For preparation of the inhaler, follow the instructions supplied with the inhaler.
1. Remove caps from the inhaler and chamber. Shake the inhaler immediately before use as per the instructions supplied with it.
2. Insert the inhaler into the backpiece of the chamber. Put mouthpiece into mouth and close your lips around it to ensure an effective seal.
3. Exhale then press the inhaler once at the beginning of a slow inhalation. Inhale slowly and deeply through the chamber until a full breath has been taken. Hold your breath for 5-10 seconds before exhaling. OR Exhale and press the inhaler once at the beginning of a slow inhalation. Breathe in and out through the chamber for 2-3 breaths keeping lips sealed around chamber mouthpiece.
Notes
· Inhalation — SLOW DOWN if you hear the whistle sound, it means you are inhaling too quickly (on adult models).
· If someone is helping you they can use the Flow-Vu* Inhalation Indicator to ensure a good seal, coordinate pressing the inhaler with inhalation and to count the number of breaths taken. The Flow-Vu* Indicator moves toward you as you inhale and only moves if you have a proper seal.
· Administer one (1) puff at a time. Follow instructions supplied with the inhaler on how long to wait before repeating.
· Any serious incident that has occurred in relation to the device should be reported to the manufacturer and the competent authority of the Member State.
Cautions
· Do not use with Dry Powder Inhalers or Nebulizers. This chamber has not been designed to deliver these medications so you may not get the proper dose.
· Failure to follow instructions may affect medication delivery or cause inhalation issues.
Warnings
Do not leave the device unattended with children. Contains small parts that can cause choking.
FOR USE WITH RESPIMAT† SOFT MIST INHALERS
For preparation of the inhaler, follow the instructions supplied with the inhaler.
1. Hold the inhaler upright and turn the clear base in the direction of the arrows on the label until it clicks. (Keep the inhaler cap closed).
2. Open the cap of the inhaler until it snaps fully open. Remove the cap from the chamber.
3. Insert inhaler into the backpiece of the chamber. Do not cover the air vents (A) on the inhaler. Place mouthpiece into mouth and close lips around it to ensure an effective seal.
4. Exhale then press the dose release button (B) on the inhaler at the beginning of inhalation. Inhale slowly and deeply through the chamber until a full breath has been taken. Hold your breath for 5-10 seconds before exhaling. OR Exhale then press the dose release button (B) on the inhaler at the beginning of a slow inhalation. Breathe in and out through the chamber for 2-3 breaths keeping lips sealed around chamber mouthpiece.
CLEANING
This chamber can be used right out-of-package and then cleaned weekly.
1. Remove the backpiece. To detach the frontpiece, twist chamber as shown.
2. Soak the parts for 15 minutes in a mild solution of liquid dish detergent and lukewarm clean water. Agitate gently. Rinse parts in clean water. OR Place parts in top rack of dishwasher. Ensure product is securely placed face up. Run the dishwasher on a normal or light cycle. Do not heat dry.
3. Shake out excess water and allow to air dry in a vertical position. Ensure parts are dry before reassembly.
4. To reassemble, fit the frontpiece on the end of the chamber and twist firmly until securely locked into position. Center the alignment feature on the backpiece with the Flow-Vu* Inhalation Indicator, as shown. Press firmly to attach the backpiece.
Notes
· Product should be replaced after 12 months of use.
· Product is free of Bisphenol A, Phthalates, Latex, Lead and PVC.
· If you notice medication build-up in your chamber, wash the inside of the chamber gently with a soft cloth.
· Dishwashing with overly dirty dishes is not recommended.
· If cleaning in a dishwasher use a rinse aid.
· For cleaning of the inhaler, follow the instructions supplied with the inhaler.
· The chamber device can be disposed of with domestic waste unless this is prohibited by the disposal regulations prevailing in the respective member countries.
· Put cap back on the mouthpiece and store in a clean, dry area when not in use.
Caution
Do not boil or sterilize. Product may be permanently damaged if boiled, sterilized or cleaned in a dishwasher at a temperature above 70°C.
Please necessary read the packaging insert before use.
Manufacturer: Trudell Medical International*, 725 Baransway Drive, London, Ontario, Canada N5V 5G4. Manufactured in Canada with Canadian and USA Parts.
Distributor : UAB „Norameda“, Meistrų g. 8A, Vilnius LT-02189. Tel. +370 5 2306499.
* trademarks and registered trademarks of Trudell Medical International (TMI). All rights reserved. © TMI 2021.
1 Lavorini et al. Targeting drugs to the airways: the role of spacer 108944-001 Rev A devices. Expert Opin Drug Deliv 2009;6(1):91-102